SAHPRA New Health Product Applications Status Checker South Africa
Organisation : SAHPRA
Facility Name : New Health Product Applications Status Checker
Country : South Africa
Website : https://www.sahpra.org.za/application-status-checker/
| Want to comment on this post? Go to bottom of this page. |
|---|
How To Check SAHPRA New Health Product Applications Status?
SAHPRA has implemented an application status checker. This new tool is listed under “Online Services” on the SAHPRA website. Accessing this tool will enable an applicant to check the status of their new health product(s) application. Please note that this tool is for Business-As-Usual applications only – and not Backlog. To Check SAHPRA New Health Product Applications Status, Follow the below steps
Related / Similar Facility : Ideal Clinic Monitoring System
Step:
1. Enter the application number for the Master application
2. Press the search button
3. View application status
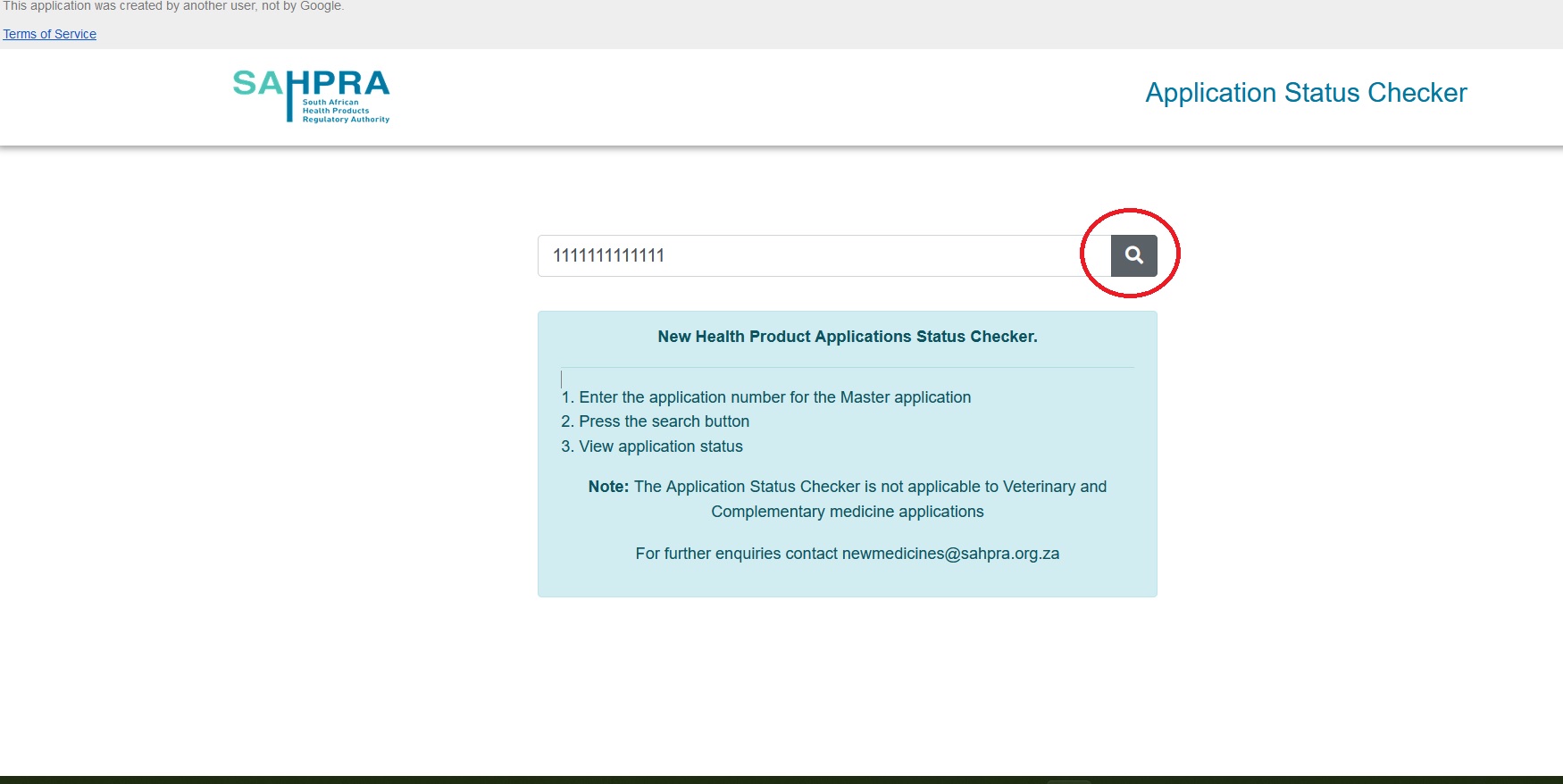
Note:
The Application Status Checker is not applicable to Veterinary and Complementary medicine applications
SAHPRA Safety Information & Updates
No therapeutic product is ever completely risk free. Some risks may be known when a medicine is first entered on the market. However, some information only comes to light after more people use the products.
This webpage was developed to provide the public and healthcare professionals with easy access to important drug safety information. The webpage contains the most recent drug safety communications from SAHPRA as well as links for early communications and follow-up.
Alerts provide important information and recommendations about therapeutic products. When an alert is issued, it does not necessarily mean a product is considered unsafe.

Know About SAHPRA:
SAHPRA is an entity of the National Department of Health, created by the South African Government to ensure that the health and well-being of human and animal health are at its core.
The Historical Trajectory:
SAHPRA assumed the roles of both the Medicines Control Council (MCC) as well as the Directorate of Radiation Control (DRC) which were housed at the National Department of Health (NDoH). Subsequently, SAHPRA was constituted as an independent entity that reports to the National Minister of Health through its Board.
Sahpra’s Core Business:
SAHPRA is tasked with regulating (monitoring, evaluating, investigating, inspecting and registering) all health products. This includes clinical trials, complementary medicines, medical devices and in vitro diagnostics (IVDs). Furthermore, SAHPRA has the added responsibility of overseeing radiation control in South Africa. SAHPRA’s mandate is outlined in the Medicines and Related Substances Act (Act No 101 of 1965 as amended) as well as the Hazardous Substances Act (Act No 15 of 1973).
SAHPRA has three pillars to ensure that medicines, medical devices and IVDs meet the requisite standards to protect the health and well-being of South Africans:
** Safety
** Efficacy
** Quality
Note:
It is these three pillars that define the ethos of SAHPRA.
Contact
For further enquiries contact newmedicines [at] sahpra.org.za